Precaution Steps in Chemistry Experiment
For labs exercises involving food or drinks lab assistants and. The document attempts to provide teachers and ultimately their students with informa-.

Titrating Sodium Hydroxide With Hydrochloric Acid Experiment Rsc Education
To this add 10 ml of 01 M KSCN using a measuring cylinder.

. Another precaution is when carrying out the titration the alkali soluti. Most of the precautions are just commonsense practices. Take 10 ml of 01 M FeCl 3 solution in a measuring cylinder and pour it into a clean beaker.
Updated and revised to reflect those changes. General Safety rules The laboratory can be but is not necessarily a dangerous place. When intelligent precautions and a proper understanding of techniques are employed the laboratory is no more dangerous than any other classroom.
However it is OK to write a few sentences on the overall operation of the experimental apparatus. 2Their should not be any leakage from the burette during titration. This guide on safety in the chemistry labo-ratory was also written to provide high school chemistry teachers with an easy-to-read reference to create a safe learning environment in the laboratory for their students.
Do not throw broken glass apparatus or used filter paper in the sink. Always wear an apron in the laboratory to protect your clothes. View Chemistry experiment precautions and steps bookdocx from CHEM 1001 at Saint Francis High School Calgary.
1Usually an air bubble is present in the nozzle of the buretteit must be removed before taking the initial reading. Before the titration process rinse the pipette and flask with the respective solutions that are going to be filled with to make sure the inner surfaces are free from any other solutions or distilled water. 3Keep your eye in level with the liquid surface while taking the burette reading or while reading the pipette or measuring flask etc.
While heating keep the mouth of test tube away from your eyes and face. The annotated diagram serves the purpose of a description of the procedure used in the experiment. Experiment 3 - Hydrocarbons Introduction Organic chemistry is the chemistry of the compounds of carbon.
No food or drink is allowed in lab unless food or drinks are provided as a part of the lab. Dilute the deep red solution by adding 50 ml of distilled water. Do not write a step-by-step procedure that mimics the steps given in the instructions.
About 90 of them are organic ie they contain carbon. A deep red colour is obtained due to the formation of the complex Fe SCN H 2 O 5 2 aq. The remaining compounds are called inorganic and are formed from the other elements of which there are.
Subjects Science Chemistry. Answer 1 of 11. Throw them in a dustbin.
Even though lab tables and counters are wiped down before each lab set up as a result of some laboratory exercises chemical residues may be present on the tables. Read the experiment carefully before coming to the laboratory. Currently over twenty million compounds have been reported in the chemical literature.
What is the precaution steps in the experiment of the determination of the molar mass of a volatile liquid. PRECAUTIONS IN TITRATION. The apparatus to be used in an experiment should be arranged neatly before beginning an experiment.
Ad Browse Discover Thousands of Science Book Titles for Less.

Safety Tips For Safe Experiments In Your Chemistry Laboratory Lab People

Chemical Traffic Light Experiment

Chemistry Laboratory Safety Rules Lab Manager
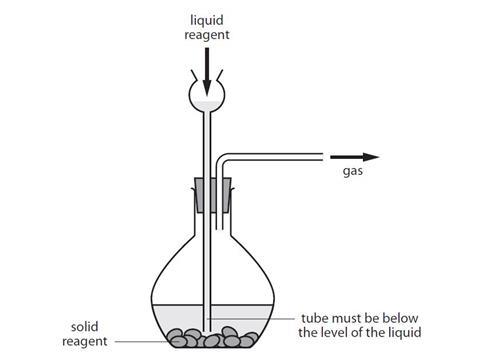
Generating Collecting And Testing Gases Experiment Rsc Education
0 Response to "Precaution Steps in Chemistry Experiment"
Post a Comment